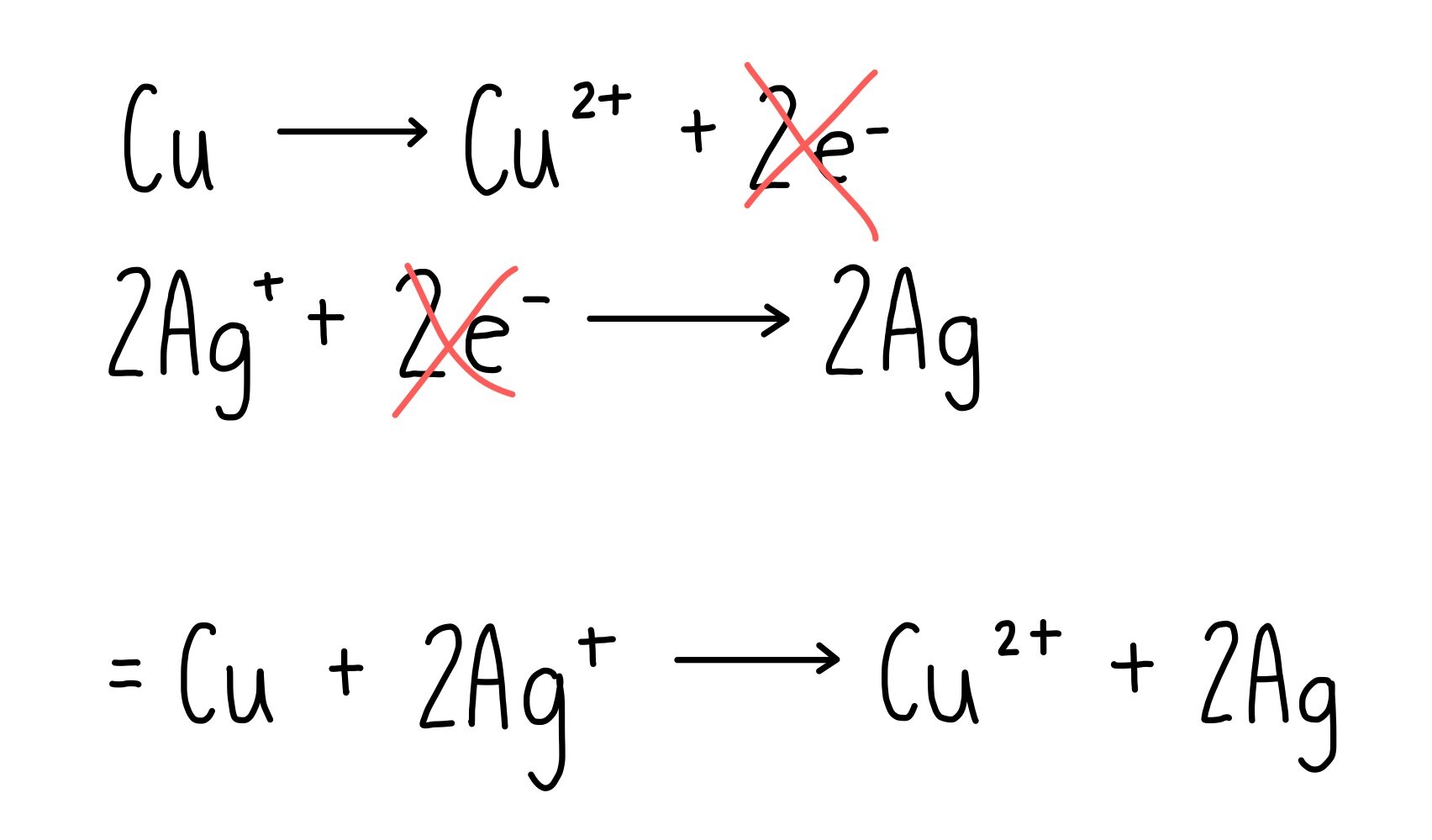Copper Cycle Equations . It states that mass is neither. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. The law of conservation of mass is one of the fundamental laws of chemistry. the different copper species obtained in each part is shown in equation 1 below: Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. cycle of chemical reactions of copper. Chemistry of copper and grouping the. In this experiment, you will carry out a series of reactions starting with copper metal. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions.
from www.thesciencehive.co.uk
The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. In this experiment, you will carry out a series of reactions starting with copper metal. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. The law of conservation of mass is one of the fundamental laws of chemistry. cycle of chemical reactions of copper. Chemistry of copper and grouping the. the different copper species obtained in each part is shown in equation 1 below: It states that mass is neither.
Redox and Electrode Potentials — the science hive
Copper Cycle Equations cycle of chemical reactions of copper. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. cycle of chemical reactions of copper. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. In this experiment, you will carry out a series of reactions starting with copper metal. Chemistry of copper and grouping the. The law of conservation of mass is one of the fundamental laws of chemistry. It states that mass is neither. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. the different copper species obtained in each part is shown in equation 1 below:
From www.chegg.com
Solved TYPES OF CHEMICAL REACTIONS AND THE COPPER CYCLE Copper Cycle Equations Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. Chemistry of copper and grouping the. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. In this experiment, you will carry out a series of reactions starting with copper metal. It. Copper Cycle Equations.
From www.homeworklib.com
Reactions and Neutralization in the Copper Cycle A Cycle of Chemical Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. It states that mass is neither. Chemistry of copper and grouping the. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first. Copper Cycle Equations.
From www.slideserve.com
PPT Copper Cycle PowerPoint Presentation, free download ID450596 Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. the different copper species obtained in each part is shown in equation 1 below: Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions.. Copper Cycle Equations.
From www.chegg.com
Solved THE COPPER CYCLE OBJECTIVE To observe chemical Copper Cycle Equations the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. It states that mass is neither. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. cycle of chemical reactions of copper. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu.. Copper Cycle Equations.
From wesleynealapchemistrylabs.weebly.com
Copper Lab wesleynealapchemistrylabs Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. It states that mass is neither. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Chemistry of copper and grouping the. Web. Copper Cycle Equations.
From www.youtube.com
How to Balance Copper plus Oxygen Gas YouTube Copper Cycle Equations Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. In this experiment, you will carry out a series of reactions starting with copper metal. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions.. Copper Cycle Equations.
From www.studocu.com
Copper report Lab report, hand in within 48 hours Copper Cycle Lab Copper Cycle Equations the different copper species obtained in each part is shown in equation 1 below: It states that mass is neither. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. The law of conservation of mass is one of the fundamental laws of chemistry. cycle of chemical reactions of copper. In this experiment, you will carry out. Copper Cycle Equations.
From www.chegg.com
Solved THE COPPER CYCLE OBJECTIVE To observe chemical Copper Cycle Equations Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. In this experiment, you will carry out a series of reactions starting with copper metal. Chemistry of copper and grouping the. cycle of chemical reactions of copper. The sequence begins with copper metal and. Copper Cycle Equations.
From studylib.net
A Cycle of Copper Reactions Copper Cycle Equations In this experiment, you will carry out a series of reactions starting with copper metal. the different copper species obtained in each part is shown in equation 1 below: Chemistry of copper and grouping the. It states that mass is neither. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of. Copper Cycle Equations.
From studylib.net
Types of Reactions The Copper Cycle Copper Cycle Equations cycle of chemical reactions of copper. the different copper species obtained in each part is shown in equation 1 below: In this experiment, you will carry out a series of reactions starting with copper metal. Chemistry of copper and grouping the. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. The sequence begins with copper metal. Copper Cycle Equations.
From www.chegg.com
Solved THE COPPER CYCLE A Series of Reactions PRELAB Copper Cycle Equations Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. Chemistry of copper and grouping the. the different copper species obtained in each part is shown in equation 1 below: The law of conservation of mass is one of the fundamental laws of chemistry. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. In. Copper Cycle Equations.
From studylib.net
Copper Cycle Lab Instructions Copper Cycle Equations cycle of chemical reactions of copper. In this experiment, you will carry out a series of reactions starting with copper metal. Chemistry of copper and grouping the. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. the different copper species obtained in each part is shown in equation 1 below: It states that mass is neither.. Copper Cycle Equations.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Cycle Equations Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves transforming cu. In this experiment, you will carry out a series of reactions starting with copper metal. The law of conservation of mass is one of the fundamental laws of chemistry. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Chemistry of copper and. Copper Cycle Equations.
From www.goldhilleducation.co.uk
IGCSE Chemistry Mychem Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. the different copper species obtained in each part is shown in equation 1 below:. Copper Cycle Equations.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Copper Cycle Equations the different copper species obtained in each part is shown in equation 1 below: the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Chemistry of copper and grouping the. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. Cu(s) part i cu2+(aq) part ii. Copper Cycle Equations.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. It states that mass is neither. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Cu(s) part i cu2+(aq) part ii cu(oh) 2 (s) part iii. Chemistry of copper and grouping the. The sequence begins with copper metal and ends with copper. Copper Cycle Equations.
From www.youtube.com
The Copper Cycle Experiment A Series of Reactions YouTube Copper Cycle Equations The law of conservation of mass is one of the fundamental laws of chemistry. the different copper species obtained in each part is shown in equation 1 below: In this experiment, you will carry out a series of reactions starting with copper metal. cycle of chemical reactions of copper. It states that mass is neither. the reaction. Copper Cycle Equations.
From www.chegg.com
Solved A CYCLE OF COPPER REACTIONS For each step of the Copper Cycle Equations The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. In this experiment, you will carry out a series of reactions starting with copper metal. the reaction series includes single replacement, double replacement, synthesis, and decomposition reactions. Oxidizing copper metal with concentrated nitric acid, hno3(aq) the first step involves. Copper Cycle Equations.